VenaSeal ‘Vein Glue’ Approved By FDA To Treat Varicose Veins
VenaSeal ‘Vein Glue’ Approved By FDA To Treat Varicose Veins
Earlier this week, The U.S. food and Drug Administration (FDA) approved the VenaSeal closure system to permanently treat varicose veins by sealing the affected superficial veins using an adhesive agent.
This treatment has been used
According to the manufacturer’s website:
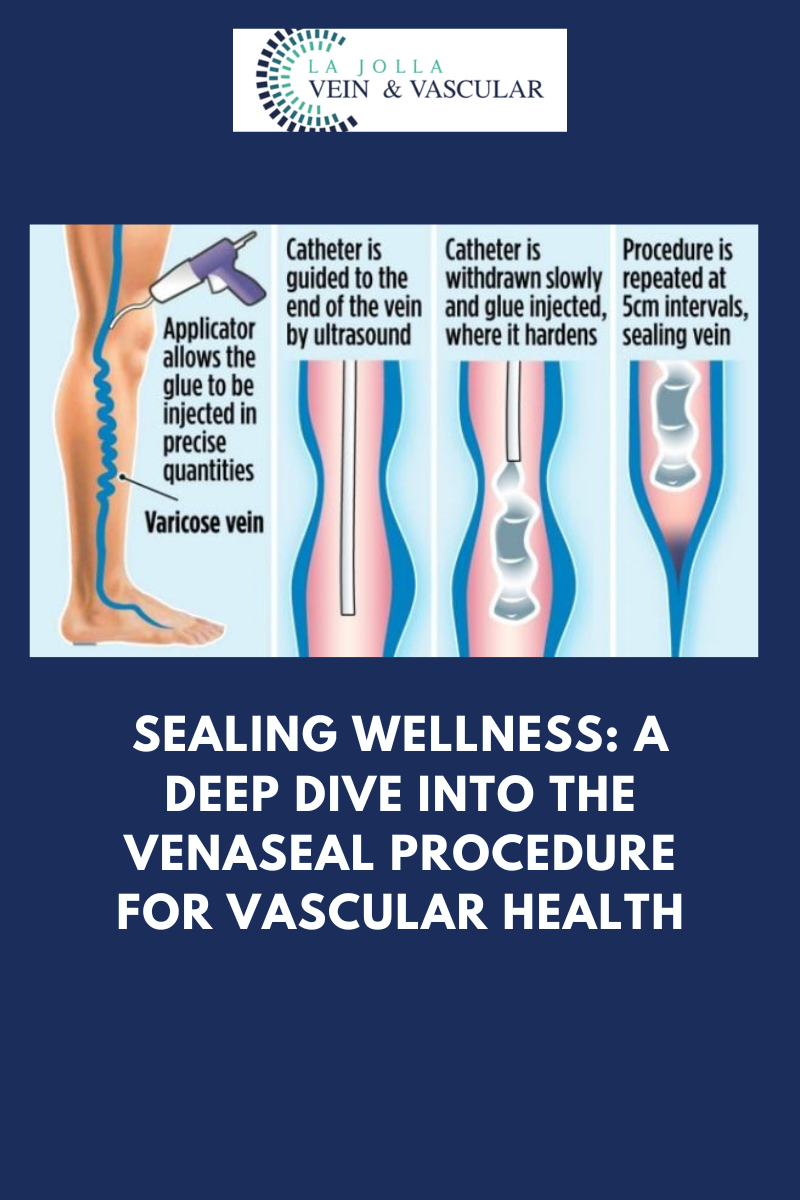
‘The VenaSeal Sapheon Closure System is a unique, minimally invasive treatment that uses a safe-for-the-body medical glue to quickly and effectively treat varicose veins (venous reflux disease). Using ultrasound, a doctor will guide a tiny catheter through a small access site in the skin and into the diseased area of the vein. Next, the VenaSeal dispenser delivers a very small amount of medical glue to close the vein. Once the affected vein is closed, blood is immediately re-routed through other healthy veins in the leg.
Unlike other treatments, VenaSeal does not require anaesthesia to be injected into the leg via multiple needle sticks (tumescent anesthesia), and because there are no pre-procedures drugs involved, patients can return to their normal activities right after the treatment. Unlike heat-based procedures, with VenaSeal there is no risk of skin burns or nerve damage. VenaSeal usually does not require any post-treatment pain medication or uncomfortable compression stockings.’